On 7
th June 2022, the “Regulatory Affairs Workshop”, co-organized by ZSHK Laboratories Limited (ZSHK) and The Hong Kong Science and Technology Parks Corporation (HKSTP), was successfully held in the Charles K. Kao Auditorium in HKSTP
The workshop aims at introducing current perspectives in the preclinical development of investigational products. Over two hundred Hong Kong biomedical company, tertiary institution and government representatives joined the workshop.

Workshop Schedule

Workshop Overview

Dr. Li Ming
Dr. Li Ming illustrated some major considerations in early druggability evaluation of chemical drugs and explained the importance of drug safety evaluation during the research and development of new drug through some typical case studies in the past 20 years.

Dr. Charles Cheung
Dr. Cheung introduced a variety of basic tactics and actions to effectively manage the quality of early phase pharmaceutical research, such as the importance of employee training, keeping records, doing data backups and following instructions.
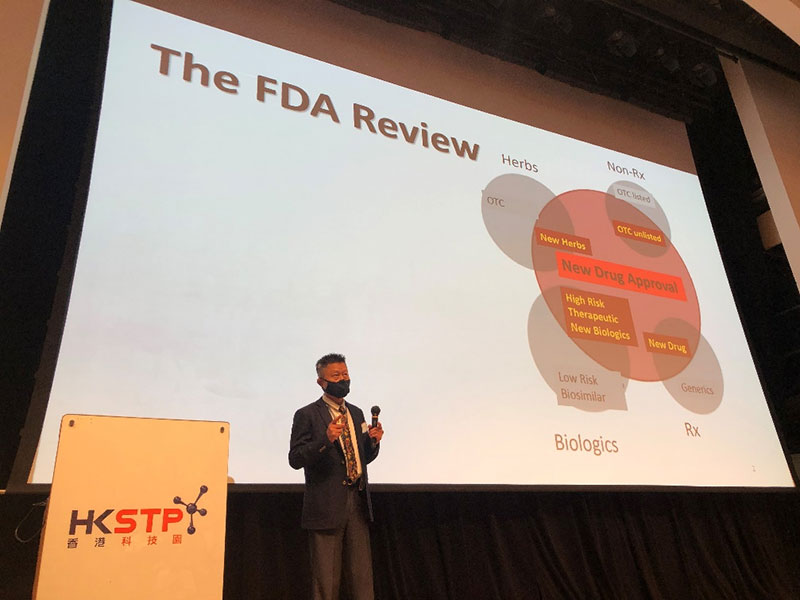
Dr. Mu Ying
Dr. Mu explained on the general requirements and key contents of filling IND application for FDA with biologics and small nucleic acid drugs as the key focus of the speech, and introduced the close relationship between biomarkers research and new drug development.

Photo with Event Representatives
(L-R: Dr. Charles Cheung, Dr. Li Ming, Dr. Grace Lau, Dr. Cecilia Pang (Biotechnology Director of ITC), Dr. Mu Ying)
This event was the first phase of “Pharmaceutical Research and Development Seminar", for the information of the remaining part of the event, please follow our company’s website(
https://www.zshklabs.com/)and WeChat media platform. Should you need any further discussion, feel free to contact our company through: Mr. Ma Jun Yi (
majunyi@anlingbio.com) (852-55979356)





