"May 17, 2023, Hong Kong, China. The 3rd Asia Medical and Health Summit (ASGH), jointly organized by the Hong Kong Special Administrative Region Government and the Hong Kong Trade Development Council, was grandly held at the Hong Kong Convention and Exhibition Centre. The forum, themed "Transforming Healthcare, Reshaping the Future," brought together major government officials and organizations responsible for healthcare from around the world, international research and medical experts, investors, as well as representatives from the business, finance, and professional service sectors of the related industries. Together, they explored the prospects and business opportunities in the medical and health industry."
ZSHK Laboratories Limited("ZSHK") was invited for the second time to participate in the ASGH conference, where they had an exhibition booth for communication purposes. The booth attracted a large number of experts and colleagues from the global biopharmaceutical field to exchange and discuss ideas together. Through ASGH, a global platform for communication, ZSHK had extensive discussions and established friendly connections with professionals from around the world, promoting research and development collaborations. ZSHK specializes in preclinical CRO services and has laboratories in Hong Kong, Shenzhen, and Suzhou. They provide non-clinical drug safety evaluations that comply with international regulations, assisting pharmaceutical companies in their research and development efforts.

Booth picture
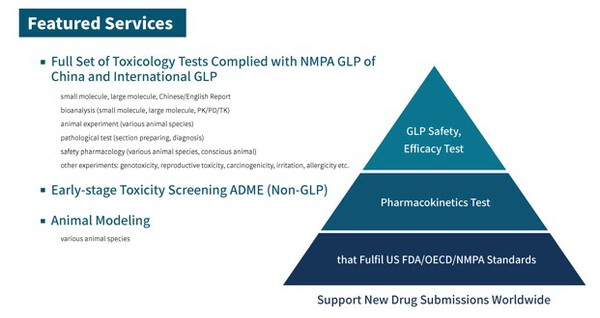
ZSHK has a preclinical GLP platform, which includes the Drug Safety Evaluation Laboratory at the Hong Kong Science Park, Anling (Suzhou), and Anling (Shenzhen). Additionally, they have monkey breeding and rearing facilities as well as dog rearing facilities, providing high-quality non-human primate and dog experimental animals. These animals have well-documented and genetically sound lineage records, along with high-end animal models. The group undertakes a complete set of new drug evaluation projects in accordance with domestic and international requirements, including drug efficacy screening, efficacy evaluation, metabolism studies, and safety evaluations. The company's main clients include new drug development companies, generic drug manufacturers, and chemical research and production companies. They provide preclinical research technical services in the fields of chemical drugs, biopharmaceuticals, traditional Chinese medicine, medical devices, cell therapy products, and chemicals.