Shanghai-Hong Kong Zhongke
Biopharmaceutical preclinical research and development service company
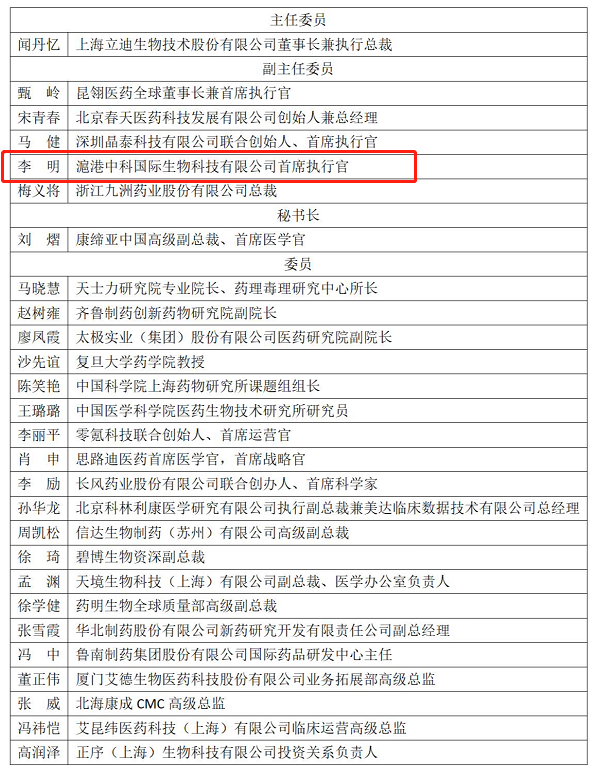
Dr. Li Ming, CEO of ZSHK, Elected as Vice Chairman of the Third Innovation and R&D Service Committee of PhIRDA
Recently, the Pharmaceutical Innovation Promotion Association (“PhIRDA") held its third-term committee re-election meeting for the Innovation and R&D Service Professional Committee ("CRO Committee") in Beijing. Dr. Li Ming, CEO of ZSHK Laboratories Limited(“ZSHK”), attended the re-election meeting.

According to the relevant provisions of the "Regulations on the Management of PhIRDA," the committee reviewed and approved a total of 27 members for the third term of the CRO Committee. Dr. Li Ming, CEO of ZSHK, was appointed as the new Vice Chairman of the committee.

Dr. Li Ming has been elected as the Vice Chairman of the third term CRO committee
President Song Ruilin highly praised the achievements and pragmatic work style of the Second Term CRO Committee. He also congratulated the successful convening of this transition meeting and extended congratulations to the newly elected members. President Song spoke highly of the committee's practical spirit.
President Song emphasized the importance of focusing on the early-stage development of pharmaceuticals and giving attention to clinical trials initiated by researchers. He urged the CRO Committee to work towards bridging the gap between pharmaceutical companies and clinical research institutions, facilitating collaboration between the two. Additionally, he stressed that the committee's efforts should prioritize quality over quantity, striving to provide high-quality services for the innovative R&D of pharmaceuticals in China.
Following this, the committee members present engaged in a lively discussion and unanimously agreed that the committee should assume the role of a bridge for communication between the industry, government, clinical and research institutions. The new term of the committee has outlined three main work plans:
1.Using the construction of a standardized biobank as a catalyst, further promote the standardization of innovative R&D.
2.Using the construction of standards for pre-clinical drug efficacy screening and evaluation models as a catalyst, continue to enhance the innovation and R&D service platform across the entire industry chain.
3.Using exchanges, interpretations, training, and experience sharing of ICH E series and CDE clinical guidance principles as a catalyst, further advance the translation and training work of pharmaceutical innovation-related guidelines.

Dr. Li Ming's Speech
With confidence in the leadership of the new term, we believe that the committee will further drive the clustering of service providers, boosting the transformation and enhancement of innovative achievements. As a vice chairman unit, ZSHK will continue to support the committee's development, providing one-stop pre-clinical CRO services, accelerating the R&D progress for pharmaceutical companies, and contributing to the innovative growth of the biopharmaceutical industry.

Group Photo